Sourcing Mangosteen Powder sounds simple — request a quote, compare pricing, and place a PO.
But anyone working in procurement or formulation knows the reality:
Two samples can look identical, yet only one will pass QC during pilot batching.
That difference rarely shows up in email conversations or supplier presentations — it shows up in the documents.
The COA, heavy metal report, microbial results, mesh size, moisture levels, and even the plant part declared on the Mangosteen powder specifications sheet determine whether the material is usable, or whether it will:
– fail during blending,
– trigger rework costs, or
– get stuck at the border during import (a common issue with Food grade mangosteen powder Canada requirements).
The first sign of a reliable Bulk Mangosteen Powder Supplier is not their MOQ or price —
it’s how confidently they can share documentation before you even request it.
So, let’s break down what matters in COAs, heavy metal tests, and supporting documents and how sourcing teams can use them to eliminate risk before releasing the PO.
What is Mangosteen Powder and why does documentation matter?
Mangosteen Powder is produced by drying and milling Mangosteen peel (pericarp). This material is valued in manufacturing because of its color, flow properties, and compatibility with plant-based formulas across nutraceutical, beverage, and personal care categories.
For companies importing Food grade mangosteen powder Canada or sourcing within the USA, supplier documentation determines whether the powder is suitable for:
– Clean-label applications
– QC approval
– Cross-border customs compliance
Two documents guide approval decisions:
– Certificate of Analysis (COA)
– Testing reports (microbial, heavy metals, and when required, Mangosteen powder pesticide residue testing)
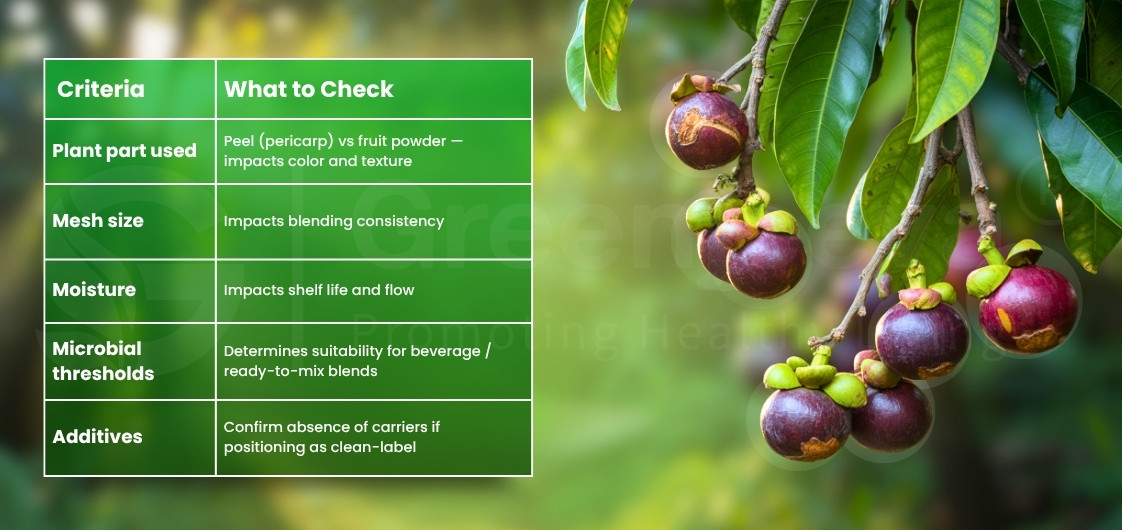
What tests must appear on a Mangosteen Powder COA?
A COA validates identity and verifies compliance with manufacturing parameters.
Typical fields include:
– Color, aroma, mesh size, and moisture (Loss on drying)
– Microbial limits (TPC, Yeast & Mold, E. coli, Salmonella)
– Heavy metal testing results (Lead, Arsenic, Cadmium, Mercury)
When evaluating a Mangosteen Powder Supplier, ensure the COA lists test methods such as USP <2021>, <2022>, <232> or equivalent. A supplier should share the Mangosteen powder specifications sheet before quote approval, not after.
How to compare COAs when evaluating Mangosteen powder suppliers?
A practical comparison method used by sourcing managers:
If multiple COAs look similar, request supporting documentation:
– MSDS
– Allergen statement
– Country-of-origin traceability
This helps differentiate a mature Mangosteen Powder Supplier from a trading intermediary.
What to look for in heavy metal and pesticide residue reports?
Mangosteen grows in tropical regions where soil naturally varies. Heavy metal results confirm whether the powder is suitable for human consumption, especially for ready-to-mix applications or beverage powders.
A compliant heavy metal tested mangosteen powder must list:
– Maximum permitted ppm per element
– Analytical method (ICP-MS or equivalent)
– Result vs. specification (Pass/Fail)
For some categories — especially Food grade mangosteen powder Canada — buyers also request pesticide residue testing to meet market-specific guidelines.
Is Mangosteen Powder clean label and how to verify it?
Instead of relying on marketing claims, verify using documents:
– Carrier disclosure (no maltodextrin, unless stated)
– GMO-free and allergen statements
– Verification that no solvents are used in processing
When “clean label” is supported by documentation, procurement approvals move faster.
Mangosteen powder benefits for formulations
Manufacturers use Mangosteen Powder because of its functionality in formulations, not due to health positioning.
Functional contributions:
– Natural color contribution (brown/purple tones)
– Powder form that supports blending consistency
– Label-friendly fruit identity
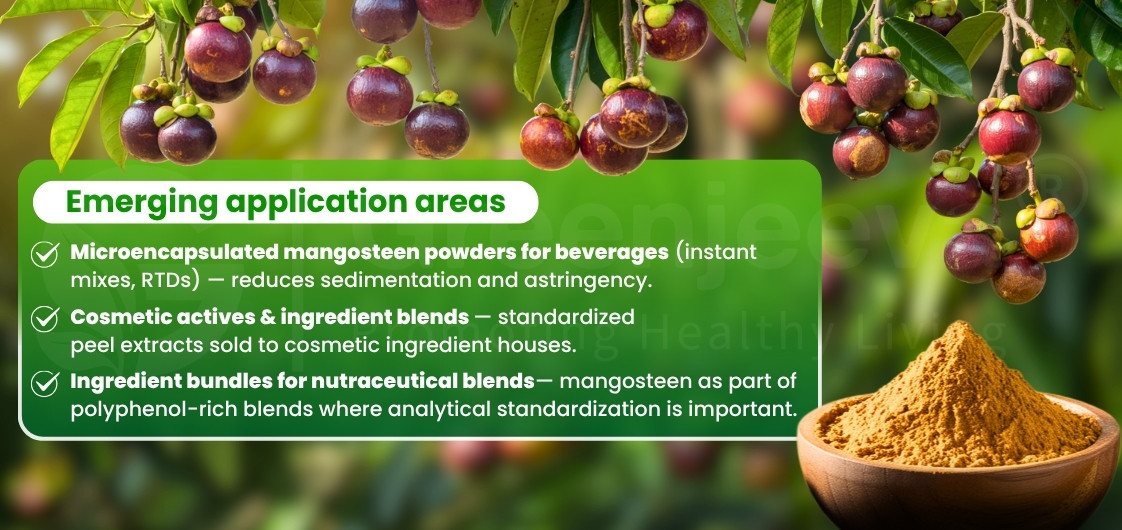
Industries using Bulk Mangosteen Powder:
– Nutraceuticals (capsules, blends, stick packs)
– Functional beverages (instant drink mixes, sachets)
– Food and beverage (fruit-based powders)
– Personal care (powdered masks, botanical scrubs)
– Pet & animal nutrition (plant-derived ingredient blends)
These are the real benefits for formulations: consistency, aesthetics, texture support, and dry-blend compatibility.
How to verify Mangosteen Powder purity before PO approval?
Before releasing a PO, procurement teams typically follow a validation sequence:
Step-by-step action plans for buyers:
1. Request Mangosteen powder specifications sheet
2. Review COA for microbial and heavy metal values
3. Request documentation packet (MSDS, Allergen, GMO, Origin)
4. Approve sample for internal lab or pilot run
For North American sourcing, this applies whether the supplier is local or a Bulk Mangosteen Powder Supplier exporting to Canada or the USA.
Best practices when qualifying a Bulk Mangosteen Powder Supplier
Select suppliers that:
– Provide free samples or pilot quantities
– Share third-party results when requested
– Support product traceability from harvest to batch
A reliable Mangosteen Powder Supplier in USA or Canada-facing exporter will provide:
– COA (batch-specific)
– MSDS
– Packaging declaration
– Country-of-origin documents
If documentation takes longer than 24–48 hours to obtain, that’s a maturity red flag.
Final Thoughts
COA and testing reports aren’t optional—they protect against:
– Batch failure during blending
– Import issues (especially Food grade mangosteen powder Canada)
– Delays in commercial scale production
Documentation determines whether Mangosteen Powder is ready for manufacturing, not the supplier’s marketing language or quoted price.
If you need compliant, documentation-ready Mangosteen Powder for commercial production:
→ Request COA and documentation packet
→ Request a sample for evaluation
→ Compare suppliers before releasing a PO
Green Jeeva supports sourcing teams with documents and sample that meet North American manufacturing requirements. Get a quote now!
**The Food and Drug Administration has not evaluated these statements. This product is not intended to diagnose, treat, cure, or prevent any disease.**