When sourcing Lactobacillus plantarum Powder 200 B CFU/g, buyers often focus on potency first. But potency alone doesn’t represent the entire quality picture. For procurement teams, formulators, and QA groups evaluating high-potency probiotic ingredients, the real question is straightforward: what must be verified before approving a supplier for bulk purchase?
This guide outlines the checkpoints that matter most when sourcing Lactobacillus plantarum 200 B CFU from a bulk probiotics supplier—practical, regulatory-aligned, and relevant to real manufacturing workflows.
What Documentation Should Buyers Check First?
Buyers typically start with documentation because it confirms compliance, identity, and consistency before product trials begin.
Documentation is the foundation of supplier qualification. Before initiating any commercial conversation, teams usually request a full technical document set to verify that the Lactobacillus plantarum powder supplier follows standard regulatory expectations.
Key documents to request include:
1. Certificate of Analysis (COA)
– Confirms the claimed potency of 200 B CFU/g at the time of production.
– Verifies microbial limits, identification, moisture content, and physical characteristics.
– Should match batch-specific data, not generic or template-based information.
2. Product Specifications
This must clearly mention:
– Strain ID and taxonomy
– Potency target: Lactobacillus plantarum Powder 200 B CFU/g
– Physical form (usually white to off-white freeze-dried powder)
– Suggested storage conditions
– Stability expectations
3. Allergen Statements
Probiotics pass through media that may contain common allergens. Verification ensures alignment with formulation restrictions and regulatory submissions.
4. Manufacturing Flowchart + Process Controls
A transparent outline of fermentation, concentration, freeze-drying, blending, and packaging helps your QA team evaluate risk points and confirm that the ingredient aligns with clean microbial handling practices.
5. Safety Certificates
– Non-GMO confirmation
– Vegan statement (if required for your product category)
– Heavy metal compliance
A reputable supplier should provide this entire set without delays or ambiguity.
How Can Buyers Verify Strain Authenticity and Identity?
Identity confirmation helps buyers ensure they are receiving the correct strain, free from cross-contamination or misidentification.
Probiotics are strain-specific. Even within the same species, functional attributes can differ significantly. Strain accuracy affects regulatory submissions, labeling, and long-term product stability.
1. Strain-Level Identification
Suppliers should provide genetic confirmation through industry-accepted methods:
– Sequencing-based verification
– Species and strain match reports
This ensures the Lactobacillus plantarum 200 B CFU bulk you receive is consistent with the declared strain.
2. Purity and Cross-Species Checks
Identity testing should confirm:
– No unwanted microbial species
– No environmental contaminants
– No mixing of closely related strains
This protects both manufacturing integrity and labeling accuracy.
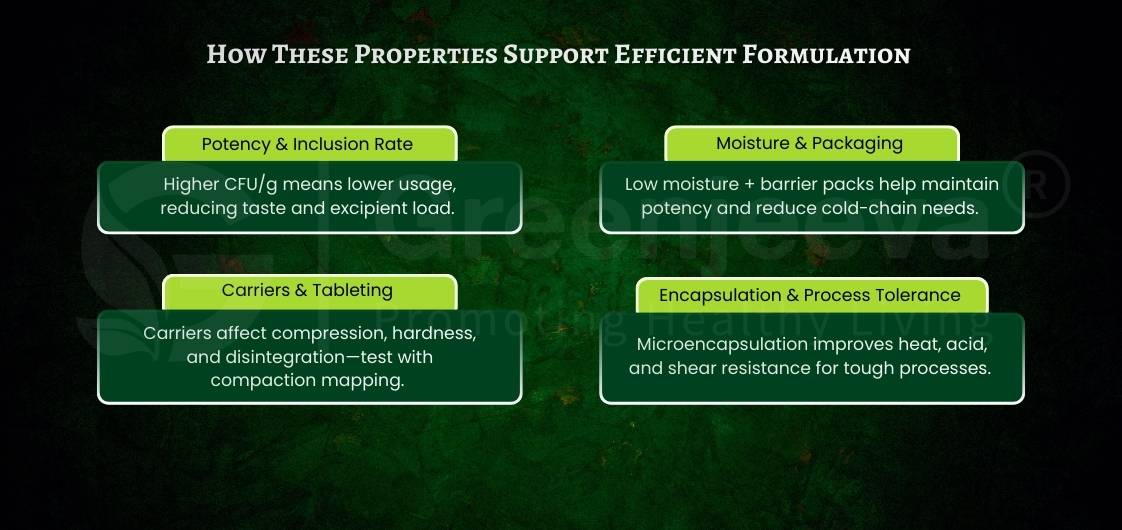
What Should Buyers Check About Probiotic Potency and Stability?
Potency claims must be supported by real stability data because CFU levels change over time and vary by storage condition.
A high number like 200 B CFU/g is compelling—but only when backed by proper QC controls.
1. Potency at Production vs. Potency at Shelf
Buyers should verify:
– The potency at the time of manufacturing
– The predicted potency at the end of shelf life
– Stability testing should mention temperature conditions and expected viability loss over time.
2. Freeze-Dried State and Moisture Levels
Moisture strongly influences the survival of freeze-dried probiotics.
Typical checks include:
– Moisture content (preferably low for stability)
– Water activity
– Packaging controls to prevent humidity exposure
3. Storage and Transport Conditions
Ensure the supplier offers:
– Cold-chain or controlled-temperature logistics
– Clear guidelines on how to store the high-potency powder upon receipt
– Timing and temperature recommendations for warehouse storage
What Quality Standards and Manufacturing Practices Should Be Verified?
Manufacturing and quality systems play a direct role in the consistency and reliability of probiotic ingredients.
Buyers must confirm the environment in which the probiotic is produced.
1. Compliance with Good Manufacturing Practices
Manufacturing should follow established hygienic and microbial handling norms. This ensures that each batch of high-potency Lactobacillus plantarum bulk powder is consistent and free from processing deviations.
2. Environmental Monitoring and Process Controls
Expect transparency about:
– Fermentation standards
– Media quality
– Sanitation processes
– Allergen control points
– Packaging cleanroom compliance
3. Consistency Controls
Ask for:
– Batch-to-batch comparison data
– Yield stability
– Historical deviation logs (if accessible)
This shows how reliably the supplier maintains quality.

What Should Buyers Validate in Terms of Microbial and Safety Testing?
Microbial testing confirms that a probiotic ingredient is safe for use in manufacturing and meets internal QA thresholds.
A comprehensive microbial report should confirm that the ingredient meets relevant safety and purity expectations.
1. Standard Microbiological Tests to Request
– Total plate count
– Yeast and mold
– Pathogen screenings
– Bile salt resistance (if the buyer requires it for internal R&D insights)
2. Heavy Metal Testing
Check compliance with standard thresholds for:
– Lead
– Arsenic
– Cadmium
– Mercury
3. Residual Solvent and Media Checks
If applicable, check whether:
– Any media components used during fermentation remain in trace amounts
– The supplier maintains proper cleaning validations
What Commercial and Operational Factors Should Be Evaluated?
Beyond technical checks, buyers also evaluate commercial consistency, supply readiness, and documentation transparency.
Probiotic sourcing decisions often depend on more than product quality. Buyers should check the supplier’s operational reliability.
1. MOQ, Lead Time, and Inventory Availability
This determines whether the ingredient can support your production planning.
2. Packaging Format
Confirm:
– Inner and outer packaging
– Oxygen barrier levels
– Sealing integrity
– Label accuracy
3. Ability to Provide Batch Consistency
Consistent supply is critical when using high-potency ingredients in capsules, blends, or dry mixes.
4. Support for R&D Trials
A supplier experienced in bulk probiotics should offer:
– Sample batches
– Technical support
– Documentation fast enough to avoid delays in formulation
Text on a green background highlights the variability of L. plantarum strains and the importance of strain designation for business.

Conclusion
Approving a Lactobacillus plantarum powder supplier requires more than checking potency figures. From strain identity and documentation accuracy to safety testing and consistency controls, each verification step plays a role in ensuring the reliability of Lactobacillus plantarum Powder 200 B CFU/g for manufacturing. When suppliers provide transparent data, well-structured documentation, and proven process controls, buyers gain clarity and confidence in long-term sourcing decisions.
If your team is evaluating Lactobacillus plantarum powder, 200 B CFU/g in bulk for upcoming formulations and needs a reliable sourcing support, Green Jeeva can assist with technical documents, batch availability, free sample, and quality verification aligned with your internal standards. Get a quote now!