Ashwagandha is one of the most widely used botanical ingredients across global nutraceutical and herbal formulations. In Canada, however, organic ashwagandha root powder is not evaluated casually.
For procurement, QA, and regulatory teams, ashwagandha is considered a high-scrutiny botanical one that requires careful validation of botanical identity, plant part, documentation, and supply reliability before approval.
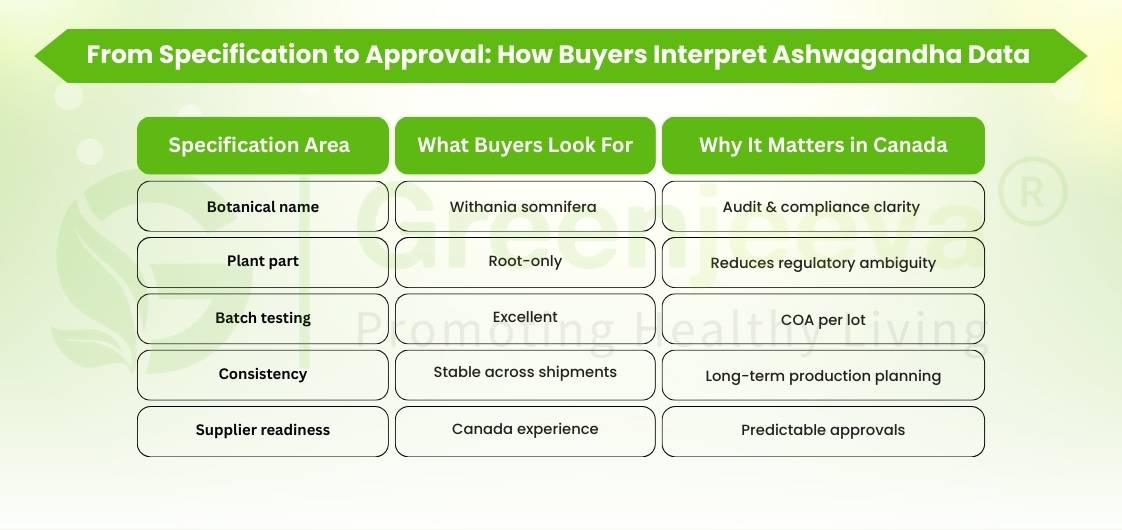
This guide outlines how Canadian buyers typically evaluate ashwagandha root powder (Withania somnifera) for commercial use.
Ashwagandha in Canada: Why Evaluation Standards Are Higher
Unlike many food ingredients, ashwagandha is a regulated botanical in Canada. Its use in finished products often triggers additional internal review compared to conventional plant powders.
As a result, Canadian buyers tend to prioritize:
– Correct botanical identification
– Root-only sourcing confirmation
– Organic certification validity
– Documentation readiness for audits
– Long-term bulk supply reliability
This makes ashwagandha a fundamentally procurement-driven decision, rather than a marketing-led one.
Why “Root Powder” Matters More Than the Name
One of the most critical distinctions made early in supplier evaluation is plant part verification.
Ashwagandha may be offered in multiple formats across the global market, including:
– Root powder
– Leaf powder
– Root–leaf blends
For Canadian buyers, ashwagandha root powder is typically preferred because:
– It aligns with established monograph expectations
– It reduces ambiguity during quality and regulatory reviews
– It supports consistent documentation and traceability
Confirming that the ingredient is root-only Withania somnifera powder is often a baseline requirement before commercial approval.

Botanical Identity and Withania somnifera Traceability
Correct botanical naming plays a central role in Canadian compliance workflows.
Procurement and QA teams usually verify:
– Latin binomial accuracy (Withania somnifera)
– Consistency of naming across specifications, COAs, and certificates
– Traceability from source to finished powder
Canadian buyers typically evaluate ashwagandha root powder, powdered ashwagandha, and Withania somnifera powder as interchangeable descriptions only when documentation clearly confirms botanical accuracy and root-only sourcing.
Discrepancies in botanical identity can delay approvals or trigger additional reviews even when the ingredient itself appears suitable.
Bulk Ashwagandha Sourcing Realities in Canada
When sourcing ashwagandha bulk powder for the Canadian market, buyers often encounter considerations beyond those seen in less regulated regions.
Common procurement realities include:
– Import and customs documentation timelines
– Batch-to-batch consistency across shipments
– Microbial and heavy metals testing expectations
– Supplier responsiveness during documentation reviews
When sourcing ashwagandha root powder bulk, Canadian buyers frequently prioritize documentation readiness and supply reliability over short-term cost advantages.
Documentation Consistency for Ashwagandha Powder
For compliance purposes, many QA teams ensure that ashwagandha powder (Withania somnifera) is labeled consistently across all technical documents, including specifications, COAs, and internal records.
Consistency in naming such as ashwagandha powder Withania somnifera helps streamline internal approvals and reduces the risk of discrepancies during audits or customer reviews.
What Canadian Buyers Typically Evaluate Before Approval
Before approving organic ashwagandha root powder for commercial use, Canadian procurement and QA teams generally assess a defined set of criteria, including:
– Organic certification validity and scope
– Root-only confirmation
– Batch-specific Certificates of Analysis (COA)
– Microbial and heavy metals test reports
– Lot-level traceability
– Supplier experience supporting the Canadian market
These checks help reduce regulatory risk and ensure continuity during scale-up.
Choosing an Ashwagandha Root Powder Supplier for Canada
There is no universal “best” ashwagandha powder. The right choice depends on:
– Intended application
– Internal compliance standards
– Production scale
– Documentation requirements
Canadian manufacturers typically review ashwagandha root powder suppliers that can demonstrate compliant bulk supply, documentation consistency, and long-term reliability before final approval.
Final Perspective
In the Canadian market, organic ashwagandha root powder is evaluated less for its popularity and more for its verifiability.
Suppliers that can support ashwagandha root powder bulk requirements with consistent documentation, botanical accuracy, and supply reliability are better positioned to meet the expectations of Canadian procurement and QA teams.