The Canadian nutraceutical market is experiencing a remarkable surge in demand for probiotics. From Toronto’s bustling supplement retailers to Vancouver’s functional beverage startups, manufacturers are constantly searching for reliable, high-quality bulk probiotic powders that meet stringent regulatory standards. Among these, Bacillus coagulans powder has emerged as a preferred ingredient for Canadian formulators. Its 200B CFU/g potency in a shelf-stable powder format makes it highly versatile across capsules, functional drinks, and fortified foods.
However, sourcing Bacillus coagulans bulk powder for Canadian applications is not simply a matter of buying in volume. Manufacturers must navigate supplier reliability, CFIA compliance, Health Canada NHP guidelines, and logistics challenges. This blog provides an insider’s guide for sourcing managers, R&D teams, and brand owners seeking Bacillus coagulans bulk supplier options that combine quality, transparency, and scalability. By the end, readers will have actionable insights for evaluating suppliers, understanding quality benchmarks, and ensuring smooth procurement for the Canadian market.
What is Bacillus coagulans in the Context of Bulk Supply?
Bacillus coagulans is a spore-forming probiotic recognized for its resilience in processing and storage. Unlike other probiotic strains, its spore form allows it to withstand heat, moisture, and pH changes—making it particularly suitable for bulk probiotic powders destined for diverse applications.
Commercial formats commonly available include:
Powder form — Standardized to 200B CFU/g for high-potency formulations.
Granular form — Used in capsule blends, tablet formulations, and functional beverage mixes.
Global sourcing origins:
India, USA, and select European regions dominate production.
Suppliers often operate under ISO, and HACCP protocols to meet international quality and regulatory standards.
Canadian buyers value not only potency and stability but also traceability. Understanding the origin of Bacillus coagulans bulk powder and its processing protocols helps ensure compliance with Health Canada and supports claims related to ingredient transparency.
Quality Benchmarks for Bulk Bacillus coagulans
High-quality Bacillus coagulans powder is more than CFU counts—it’s a combination of purity, stability, and compliance. Canadian formulators typically evaluate suppliers based on the following benchmarks:
Potency Verification – Ensure Bacillus coagulans 200B CFU/g is verified by independent labs at the time of manufacture and projected shelf life.
Moisture Content – Ideally ≤5% to maintain stability during storage and transportation.
Mesh Size & Particle Uniformity – Critical for consistent blending into powders, capsules, or beverage mixes.
Shelf Stability – Confirmed through validated studies; spore-forming probiotics like Bacillus coagulans maintain viability at ambient storage conditions.
Certifications – GMP, HACCP, Kosher, Halal, Non-GMO, and Canada Organic certifications are crucial for market acceptance.
Testing Protocols – Routine microbial assays, heavy metal analysis, and allergen verification.
Documentation – COA, MSDS, regulatory compliance certificates, and traceable batch information.
Tip: For Canadian markets, ask suppliers about NHPID registration and adherence to Health Canada guidelines for finished products. A supplier who can provide robust documentation and third-party testing demonstrates credibility as a certified Bacillus coagulans powder source.

Sourcing Insights & Supply Chain Transparency
Securing a consistent supply of Bacillus coagulans bulk USA or global sources requires understanding the broader supply chain.
Production hubs & lead times:
India: Large-scale production, cost-effective, shipping lead time 4–6 weeks via sea freight.
USA: Smaller batch sizes, premium quality, faster shipping, suitable for Canadian importers.
Europe: Specialized formulations and certifications for clean-label or organic products.
Shipping & storage considerations:
Cold chain shipping is rarely necessary for Bacillus coagulans, but ambient temperature stability data is essential.
Warehouse placement in Canada (Western vs. Eastern provinces) can impact lead times for national distribution.
Sustainability & traceability:
Canadian buyers increasingly prioritize sustainable cultivation practices and transparent sourcing. Suppliers offering full traceability, eco-friendly packaging, and ethical manufacturing processes have a competitive edge in the Canadian nutraceutical space.
Applications in Food & Nutraceutical Formulations The versatility of Bacillus coagulans for dietary supplements extends across multiple categories:
The versatility of Bacillus coagulans for dietary supplements extends across multiple categories:
Capsules & Tablets – Incorporated into multi-strain probiotic formulations.
Functional Beverages – Shelf-stable powders are ideal for RTDs and plant-based drinks.
Fortified Foods – Protein bars, snack mixes, and powdered drink formulations.
Pet Nutrition – Added to supplements for gut health and immunity support.
Market trends show Canadian consumers demand clean-label, non-GMO, and shelf-stable probiotics, which aligns perfectly with the properties of Bacillus coagulans bulk powder. Manufacturers can leverage these traits to formulate products that resonate with wellness-conscious buyers.
Bulk Procurement Tips for Canadian Buyers
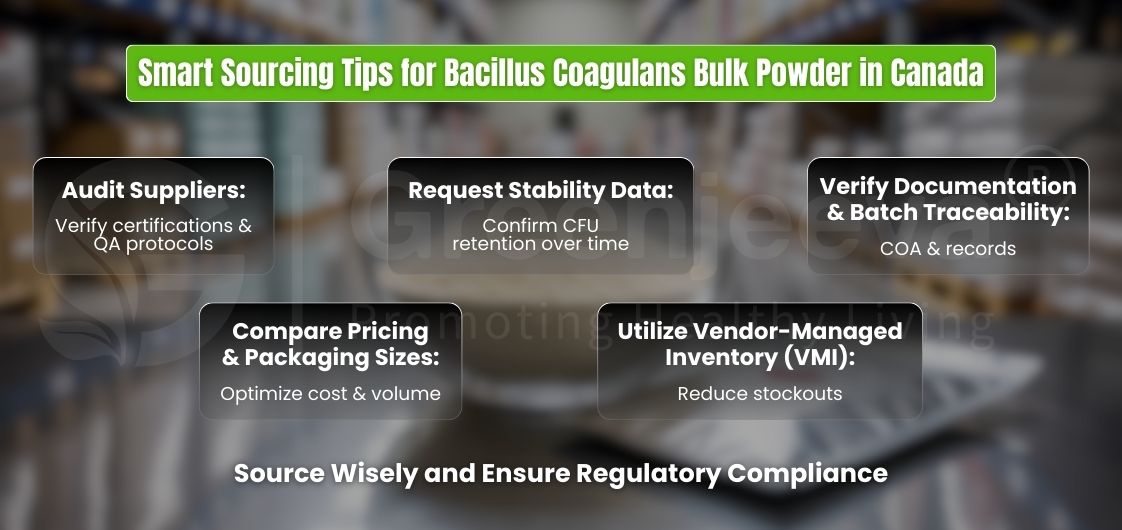
Sourcing Bacillus coagulans bulk powder efficiently requires a combination of quality evaluation, documentation scrutiny, and logistical planning:
Audit Suppliers – Review facility certifications, QA protocols, and prior customer references.
Request Stability Data – Verify potency retention over storage and transportation conditions.
Compare Pricing Factors – Consider origin, grade, certification costs, packaging sizes (10kg, 25kg, 100kg), and freight charges in CAD vs. USD.
Use Vendor-Managed Inventory (VMI) – Reduce stockouts and optimize cash flow for seasonal demand spikes.
Documentation Rigor – Ensure COA, allergen reports, and batch traceability meet Canadian regulatory requirements.
Following these steps ensures Canadian nutraceutical brands partner with a reliable bulk probiotic powders supplier who delivers both quality and compliance.
Conclusion
For Canadian nutraceutical brands, sourcing Bacillus coagulans bulk powder requires balancing quality, compliance, and supply chain transparency. From verifying 200B CFU/g potency to checking certifications and supplier reliability, every step ensures a smooth formulation process and regulatory confidence. By partnering with a trusted and certified Bacillus coagulans ingredient supplier, Canadian brands can confidently integrate this versatile probiotic into capsules, beverages, and fortified foods, while maintaining high standards and meeting consumer expectations.
Disclaimer
This article is intended for educational and industry insight purposes only. It does not provide medical advice or make health-related claims.