From protein powders to herbal compacts, the clean-label movement has transformed into a widespread purchasing standard. Today’s health-conscious consumers inspect every detail of the ingredient panel, looking for naturally derived, minimally processed, and easily recognizable components. Anything synthetic, chemically altered, or vaguely labeled is likely to raise concerns or deter purchases.
This scrutiny creates a challenge for nutraceutical product developers: how can they maintain the high performance required for tablet and capsule production while also aligning with clean-label expectations?
The answer often lies in the choice of excipient. Among the most reliable is Microcrystalline Cellulose (MCC) especially USP 101 and USP 102 grades. As a plant-based binder, MCC offers the ideal balance between formulation integrity and label-friendly transparency. It is widely used in natural product formulations due to its exceptional compressibility, inert nature, and status as a non-synthetic additive.
MCC supports a range of solid-dose formats and performs key functional roles without complicating the ingredient list. It embodies the promise of clean-label supplement innovation: delivering technical efficiency and natural authenticity in equal measure. Brands focused on ethical, safe, and effective formulations can rely on MCC as a foundational ingredient that meets both manufacturing and marketing needs.
What Is Microcrystalline Cellulose and How Is It Classified?
Microcrystalline Cellulose is a refined cellulose material derived from natural plant sources such as wood pulp or cotton linters. It undergoes a controlled process of mechanical treatment and partial hydrolysis to produce a white, odorless, tasteless powder with excellent functional performance. Its inert nature and flow-enhancing qualities make it a go-to tablet compression aid and capsule filler in the nutraceutical sector.
Crucially, MCC is not chemically modified, which preserves its status as a label-friendly excipient. It’s included in major pharmacopeias such as USP and FCC, confirming its safety, purity, and quality for both pharmaceutical and dietary supplement use.
MCC is typically available in multiple grades, with USP 101 and USP 102 being the most prevalent in supplement formulations:
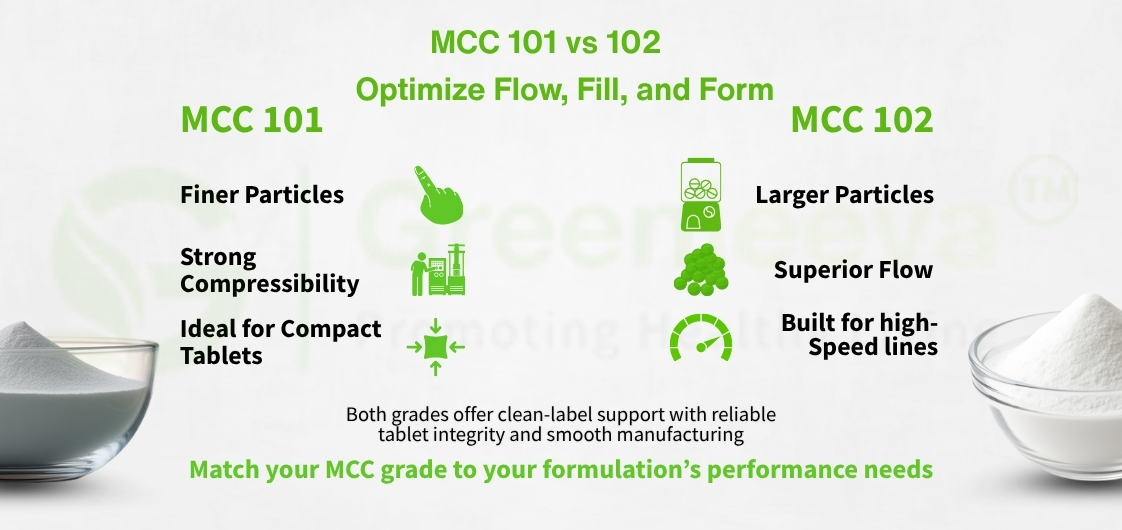
USP 101 has a finer particle size that offers superior compressibility, making it ideal for direct compression tablet manufacturing.
USP 102 features a larger particle size and better flowability, making it suitable for high-speed encapsulation equipment and powder blends.
Each grade offers advantages depending on production conditions. MCC 101 is often selected when strong tablet compaction is needed, while MCC 102 is used to prevent blend segregation during high-throughput production. Both support natural excipient systems while enhancing manufacturing efficiency.
Functional Benefits in Clean-Label Supplement Formulation
Beyond label simplicity, MCC plays a critical functional role in formulation performance. It serves as a tablet binder, disintegration agent, filler, and flow enhancer all while maintaining its status as a non-synthetic, plant-derived ingredient. This makes MCC a valuable asset for companies aiming to deliver consistent, safe, and efficient products that meet clean-label guidelines.
In tablet applications, MCC enhances compressibility, helping maintain tablet shape and structure during high-speed compression. It prevents issues like capping, cracking, and friability common concerns in botanical and high-fiber formulations. Additionally, MCC promotes uniform active distribution, even when actives are used in low concentrations.
In capsule filling, MCC serves as a bulking agent that supports accurate weight distribution. This is essential for micronutrient blends, herbal extracts, or potent actives that require precision in every capsule. MCC 102 is especially favored for its flow agent properties that improve die fill and reduce machine downtime.
Importantly, MCC does all this without contributing any taste, color, or off-notes making it perfect for sensitive blends. It is also compatible with sweeteners, flavors, and other excipients, further extending its versatility in clean-label supplement formulation strategies.
Why MCC Excels in Clean-Label Positioning
Today’s ingredient-savvy consumers are more likely to trust products that don’t require a science degree to understand. MCC fits perfectly within this consumer mindset. It is free from E-numbers, contains no synthetic chemicals, and can be simply labeled as “Microcrystalline Cellulose” on product packaging no footnotes or clarifications required.
Furthermore, MCC aligns with a wide range of certifications and dietary standards:
Vegan and vegetarian compliant
Non-GMO
Gluten-free
Allergen-free
Kosher and halal certified
This makes it a favored option for brands looking to cater to multiple dietary and ethical preferences while preserving clean-label credibility. Unlike other excipients that may require explanation or justification, MCC builds trust by virtue of its simplicity.
It’s also highly synergistic with other label-friendly binders such as acacia gum, inulin, or rice hull fiber, allowing formulators to create multi-excipient systems that are both functional and clean. MCC’s compatibility with these ingredients opens doors for innovation in organic, allergen-free, or food-grade supplement lines.
Finally, MCC suppliers are increasingly offering traceability, FSC-certified sourcing, and environmentally conscious documentation, helping brands tell a sustainability story that complements their clean-label positioning.
Key Applications in Nutraceuticals
MCC’s neutral nature, stability, and versatility make it suitable across a broad spectrum of nutraceutical formats. Here are some of its most common applications:
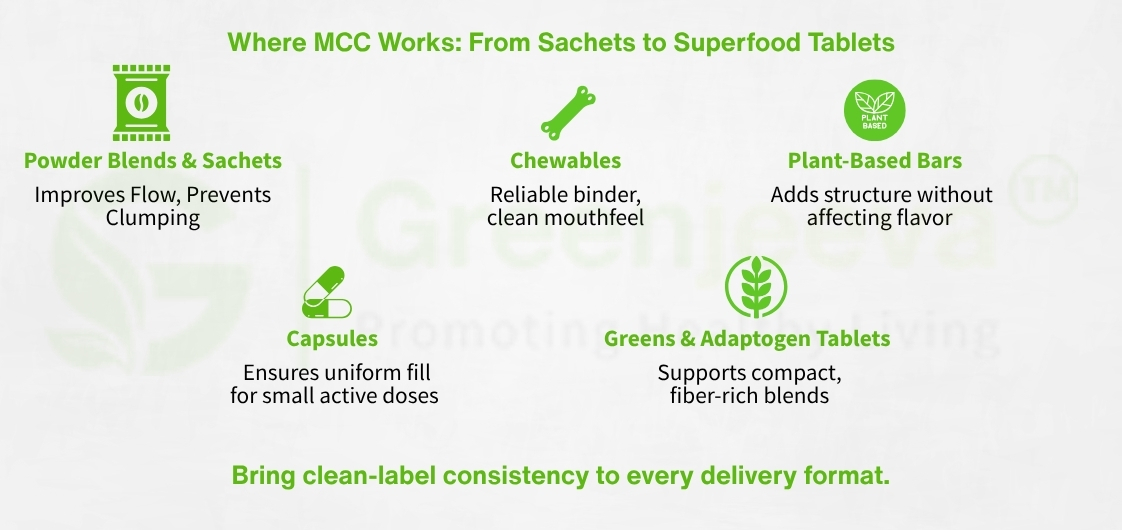
Capsule Powders and Blends: MCC 102 ensures smooth powder flow and uniform capsule weight. It supports low-dose ingredients, making it ideal for herbal or vitamin blends.
Direct Compression Tablets: MCC 101 provides excellent binding strength and compaction characteristics, reducing the need for synthetic binders or wet granulation.
Fiber-Rich Blends and Superfood Compacts: MCC stabilizes high-fiber or plant-based powders like spirulina, moringa, or adaptogens into cohesive tablets without affecting taste or appearance.
Trending Wellness Products: From greens tablets and energy blends to nootropics and gut-support tablets, MCC allows manufacturers to scale up formulations without compromising on label transparency or dosage precision.
In flavored chewables, MCC maintains texture while preventing hygroscopic degradation. In pressed powders and sachets, it improves blend uniformity and stability during shipping and storage. Its inert profile makes it particularly useful for customized nutrition packs and personalized wellness formats, where consumer scrutiny is at its highest.
What to Look for When Sourcing MCC 101 or 102
When sourcing MCC, technical specs and documentation are just as important as pricing. A reliable supplier should offer more than just product they should provide performance insights and regulatory readiness.
Here’s what to consider:
Compliance: Ensure alignment with USP monographs, FCC specifications, and international food-grade certifications.
Particle Distribution: Know whether MCC 101 or 102 fits your system based on compression behavior or flowability.
Batch Consistency: Look for historical data on compressibility, moisture content, and bulk density.
Certifications: Confirm Non-GMO, allergen-free, ISO, kosher, halal, and any sustainability or FSC documentation.
Support Services: Request COAs, MSDS, traceability logs, and sample support for formulation trials.
Additionally, working with a supplier who understands nutraceutical manufacturing environments ensures better alignment between ingredient performance and production efficiency. Some even offer prototype consultation, helping you validate MCC’s behavior before scaling.
Reliable MCC sourcing means fewer production issues, reduced need for rework, and higher batch acceptance rates outcomes that matter in competitive markets and fast-moving wellness trends.
Conclusion
In a marketplace where transparency, traceability, and technical performance must go hand-in-hand, Microcrystalline Cellulose 101 and 102 prove that excipients can support both the formulator and the consumer. They offer performance-driven functionality in tablet strength, capsule uniformity, and processing flow without adding complexity to the label.
As brands scale and innovate, MCC remains one of the most reliable formulation foundations available. It ensures integrity in clean-label supplement development, where technical challenges often collide with marketing demands. Whether you’re launching a new wellness line or updating a legacy formulation, MCC offers trust, consistency, and efficiency in a single, naturally derived ingredient.
It’s not just what MCC brings it’s what it avoids. No chemical processing, no consumer confusion, no compromise. That’s what makes MCC the backbone of clean-label success in modern nutraceuticals.