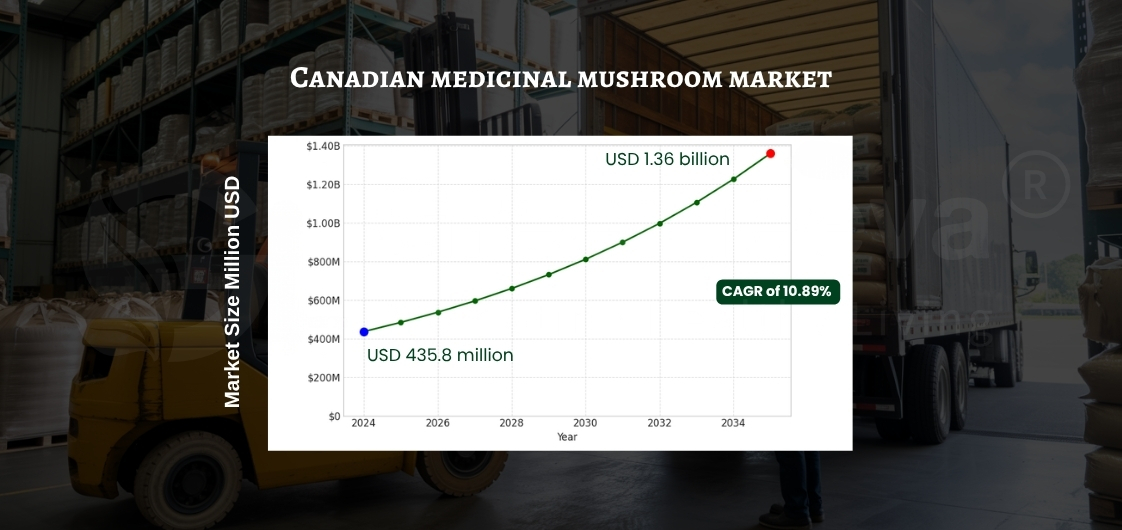
The Canadian medicinal mushroom market is experiencing significant growth, with projections indicating an increase from USD 435.8 million in 2024 to USD 1.36 billion by 2035, reflecting a compound annual growth rate (CAGR) of 10.89%. Within this expanding sector, Organic Maitake Mushroom Powder stands out as a versatile ingredient, sought after across various industries, including nutraceuticals, functional foods, personal care, and pet nutrition.
For procurement managers, sourcing heads, and R&D teams, ensuring the quality and consistency of Organic Maitake Mushroom Powder is paramount. This involves meticulous attention to sourcing practices, processing methods, and supplier certifications.
In the following sections, we delve into the critical aspects that Canadian manufacturers should consider when sourcing this ingredient in bulk.
What Defines a High-Quality Organic Maitake Mushroom Powder
Formulators often ask what distinguishes a reliable Organic Maitake Mushroom Powder from an inconsistent one.
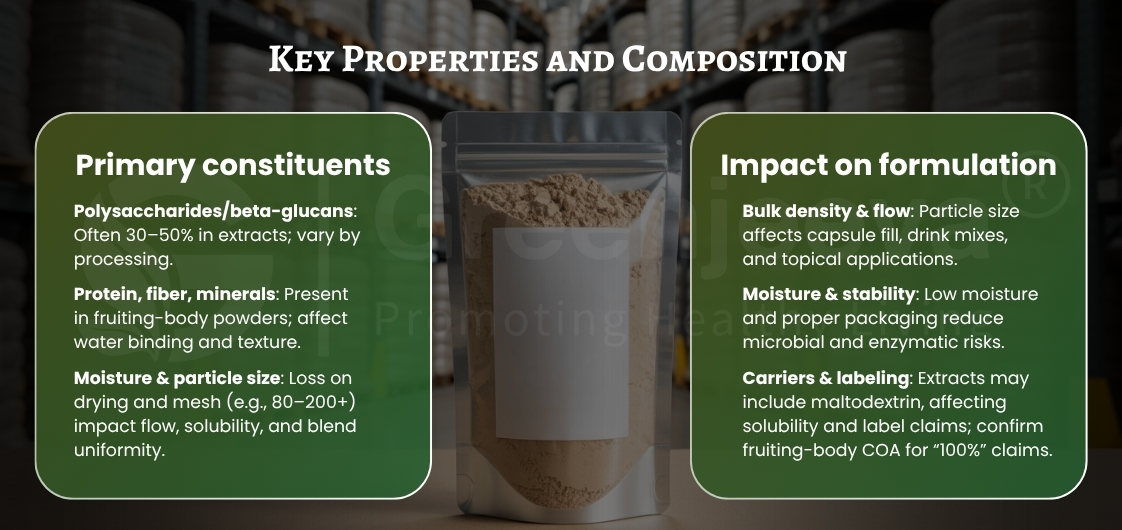
The key differentiator starts with the raw material identity. Authentic powders are derived from the fruiting body of Grifola frondosa—the portion of the mushroom that naturally accumulates polysaccharides and fiber. Some suppliers also offer mycelium-based powders grown on grain substrates; while usable, these may contain residual grain and differ in composition. Buyers should always confirm the origin declaration to match their formulation intent.
Next comes processing integrity. Clean-label production typically involves washing, controlled drying at low temperatures, and fine milling to achieve uniform mesh size. Proper dehydration helps maintain the product’s natural composition while supporting flow and shelf life.
Lastly, consistent batch testing defines long-term reliability. Each lot should demonstrate alignment with internal specs for moisture, ash, microbial limits, and heavy metal levels. Without those baselines, maintaining consistency across manufacturing runs becomes difficult.
How to Verify Sourcing and Processing Standards
Procurement teams often search for how to evaluate the sourcing chain behind an organic ingredient.
In Canada, the term “organic” carries regulatory weight under the Canada Organic Regime (COR). Suppliers must hold valid certification from a CFIA-accredited body, ensuring that the cultivation and handling meet COR standards. Requesting this certificate is non-negotiable for import or use in finished organic products.
Beyond certification, understanding processing transparency matters. Responsible suppliers disclose their dehydration and milling methods, as well as whether any carrier agents are used. Some extract powders contain maltodextrin or other carriers, which can influence label declarations and dispersibility.
For consistent sourcing, prioritize suppliers who provide full documentation—organic certificate, country of origin, and traceable lot codes—along with packaging details. Such clarity prevents compliance delays during audits or customs checks.
What Specifications Should Buyers Review Before Bulk Purchase
Formulators and sourcing professionals frequently ask what to include in technical verification before confirming a bulk order.
A reliable Certificate of Analysis (COA) is the starting point. It should list:
– Moisture (loss on drying)
– Particle size or mesh
– Ash percentage
– Polysaccharide or beta-glucan content (if specified)
– Microbiological results (TPC, yeast and mold, E. coli, Salmonella)
– Heavy metal results for lead, arsenic, cadmium, and mercury
Additional documents like MSDS/SDS, Allergen Statement, and Pesticide Residue Report should accompany the COA, especially for organic claims. For products grown on grain substrates, allergen disclosure is essential to maintain transparent labeling.
Batch-to-batch consistency can be verified through sample retention and periodic third-party testing. This process builds internal assurance that the ingredient performs predictably over time.
How Is Organic Maitake Mushroom Powder Used Across Industries
R&D teams often want to understand how this ingredient fits into various formulation systems.
– Nutraceuticals and Dietary Supplements: Used in powder blends, capsules, and tablets. Particle size uniformity supports better fill weights and reduced segregation.
– Functional Foods and Beverages: Provides natural umami notes and body to soups, drink mixes, or meal-replacement products. For beverages, finer mesh improves dispersion and mouthfeel.
– Personal Care Formulations: Applied in powdered masks or as part of extract bases for water-soluble emulsions.
– Animal Nutrition and Pet Products: Incorporated into kibble and treat formulations, subject to feed-grade documentation.
Each application has different technical requirements, from solubility to stability under heat. That’s why reviewing processing and mesh data during sourcing prevents post-formulation surprises.
What Are the Common Sourcing Challenges and How to Address Them
Many procurement managers in Canada ask how to manage sourcing risks with imported organic mushrooms.
Challenges often involve supply chain traceability and testing integrity. Mushrooms can naturally accumulate heavy metals depending on soil or substrate. Reliable suppliers counter this by testing every batch and providing recent lab reports from accredited facilities.
Another consideration is moisture control. High moisture can lead to caking or microbial growth during storage. Buyers should verify packaging methods—vacuum-sealed or double-lined drums help maintain low water activity.
Finally, minimum order quantities (MOQs) and lead times can differ between suppliers. Some operate on 25 kg drum minimums, while others handle palletized loads. Request sample lots before placing a large order and compare results under your specific process conditions.

How Does the Market Outlook Support Bulk Procurement Decisions
Industry sourcing teams often ask about market direction before committing to larger contracts.
The functional mushroom segment in Canada has been expanding steadily, with growing interest from supplement, food, and cosmetic formulators. Organic Maitake Powder, though niche compared to shiitake or reishi, is now recognized for its formulation versatility and alignment with clean-label consumer expectations.
In B2B settings, suppliers offering standardized composition and transparent documentation are being favored over unverified bulk traders. This trend suggests continued consolidation toward reliable, audit-ready sources. For sourcing managers, building supplier partnerships with consistent QA frameworks will provide better cost predictability than short-term spot purchasing.
What Steps Should Teams Take to Maintain Consistency After Purchase
Once bulk supply is secured, how can teams sustain quality through production?
Implementing internal verification protocols ensures incoming material matches the approved specification. Each new batch should be inspected for visual appearance, odor, and flow before release to production.
Proper storage control—cool, dry conditions with desiccant protection—minimizes variation in moisture and texture. For sensitive applications like drink mixes, using nitrogen-flushed or vacuum-sealed containers maintains stability longer.
Maintaining supplier communication and reviewing COAs with every shipment are practical measures that keep ingredient consistency aligned with your finished product standards.
Conclusion
Buying Organic Maitake Mushroom Powder for industrial use is no longer about simply finding a supplier—it’s about verifying a system. From certification and testing transparency to moisture control and mesh specification, every technical detail matters when producing at scale.
Canadian formulators, sourcing teams, and R&D professionals can ensure reliable performance and compliance by prioritizing verified documentation, consistent COAs, and suppliers with traceable, audit-ready processes.
Green Jeeva supports Canadian manufacturers with compliant, lab-tested, and traceable organic ingredients.
If your team is sourcing bulk Organic Maitake Mushroom Powder and needs verified specifications, you can connect with Green Jeeva’s supply experts to review documentation or request a certified sample lot for pilot evaluation.
**The Food and Drug Administration has not evaluated these statements. This product is not intended to diagnose, treat, cure, or prevent any disease.**