Sourcing Organic Thyme Leaf Powder in Canada requires careful evaluation beyond price alone. Procurement managers, sourcing heads, and R&D teams must assess quality, regulatory compliance, and supply chain reliability to secure a consistent and clean-label ingredient supply. Bulk Organic Thyme Leaf Powder is widely used across nutraceuticals, functional foods, beverages, cosmetics, and pet nutrition, so understanding sourcing considerations and formulation requirements is critical for operational efficiency.
This guide outlines key factors to consider for industrial procurement, supplier evaluation, and formulation readiness.
What Certifications Are Essential for Bulk Organic Thyme Leaf Powder?
Ensuring compliance begins with verifying certifications such as COR or USDA Organic, Non-GMO, Kosher, Halal, and Gluten-Free, which confirm the thyme powder meets regulatory standards, clean-label requirements, and consistent quality for bulk procurement.
Key certifications include:
– Canadian Organic Regime (COR) or USDA Organic – Confirms the product is grown and processed according to organic farming standards without synthetic pesticides or fertilizers.
– Non-GMO Organic Thyme Leaf Powder certification – Supports clean-label formulations for manufacturers seeking GMO-free ingredients.
– Kosher and Halal (if relevant) – Important for meeting dietary-specific requirements.
– Gluten-Free Certification – Ensures suitability for products targeting gluten-sensitive consumers.
Checking for these certifications before bulk purchase reduces the risk of non-compliance, supports labeling accuracy, and helps manufacturers meet regulatory expectations across multiple industries.
How Should Procurement Teams Evaluate Supplier Documentation for Organic Thyme Leaf Powder?
Procurement teams should carefully review COAs, MSDS, heavy metal and pesticide reports, and allergen statements to verify batch quality, safety compliance, and consistency when sourcing bulk Organic Thyme Leaf Powder for industrial use.
– Certificate of Analysis (COA) – Confirms key attributes such as moisture content, residual solvents, heavy metals, and absence of contaminants. Batch-to-batch consistency in COAs is critical for large-scale production.
– Material Safety Data Sheet (MSDS) – Offers guidance on handling, storage, and occupational safety for production teams.
– Heavy Metal and Pesticide Residue Reports – Ensures the product meets safety limits for contaminants like lead, arsenic, cadmium, and mercury.
– Allergen Statements – Verifies absence of unintended allergens and cross-contamination.
A thorough review of these documents allows sourcing teams to compare suppliers accurately and make informed decisions on bulk purchasing. Documentation transparency also streamlines internal quality audits and regulatory inspections.
What Moisture Levels Support Stability and Shelf Life in Bulk Organic Thyme Leaf Powder?
Maintaining moisture below 10% is crucial for microbial stability, extended shelf life, consistent powder flow, and reliable performance in bulk industrial formulations.
Maintaining low moisture levels:
– Reduces microbial growth risk.
– Extends shelf life during transport and storage.
– Preserves powder flowability and texture for automated processing.
Procurement teams should confirm moisture levels in the COA and, if possible, conduct random checks on new batches. Proper moisture management is especially critical when integrating the powder into powdered blends, functional foods, or beverage formulations.

How Can Bulk Supply Options Affect Procurement Efficiency?
Selecting appropriate bulk sizes, MOQs, and packaging formats ensures efficient handling, reduces waste, and aligns inventory with production schedules.
– Minimum Order Quantities (MOQ) – Bulk Organic Thyme Leaf Powder is typically offered in 10–25 kg bags, though some suppliers can provide larger or split shipments based on production schedules.
– Custom Packaging – Some suppliers accommodate private-label packaging or warehouse-ready formats to minimize handling steps.
– Storage Practices – Store in cool, dry, and airtight conditions to prevent moisture absorption and maintain product integrity.
Understanding supply options helps procurement teams plan inventory levels, reduce waste, and align bulk deliveries with production timelines. Comparing MOQs and packaging choices across multiple suppliers can also support cost efficiency and operational flexibility.
How Does Organic Thyme Leaf Powder Support Clean-Label and Formulation Requirements?
The powder’s natural processing enhances label transparency and formulation versatility.
Organic Thyme Leaf Powder is produced through drying and grinding without synthetic additives, preservatives, or fillers. Its characteristics make it suitable for:
– Functional Beverages and Herbal Blends – Adds aroma and flavor without requiring complex formulations.
– Cosmetics and Personal Care Products – Fine texture allows even integration into scrubs, masks, and powders.
– Pet Nutrition – Compatible with natural, plant-based formulations for supplements and dry food blends.
By using a single-ingredient powder, manufacturers simplify labels, reduce formulation complexity, and maintain consistent sensory qualities such as aroma, color, and texture.
What Should Procurement Managers Assess Regarding Supplier Reliability?
Procurement managers should evaluate lead times, batch consistency, traceability, and communication responsiveness to ensure uninterrupted supply and dependable quality from bulk Organic Thyme Leaf Powder suppliers.
– Lead Times and Inventory Consistency – Ensures uninterrupted production, especially for seasonal demand spikes.
– Traceability and Sourcing Practices – Confirms the thyme’s origin and compliance with organic standards.
– Batch Consistency – Review COA trends to ensure quality uniformity across shipments.
– Supplier Support – Prompt communication for documentation, shipping updates, and technical inquiries is crucial.
Evaluating supplier reliability helps mitigate risks related to production delays, regulatory compliance, and formulation quality. Multiple supplier comparisons can provide contingency options for critical production periods.
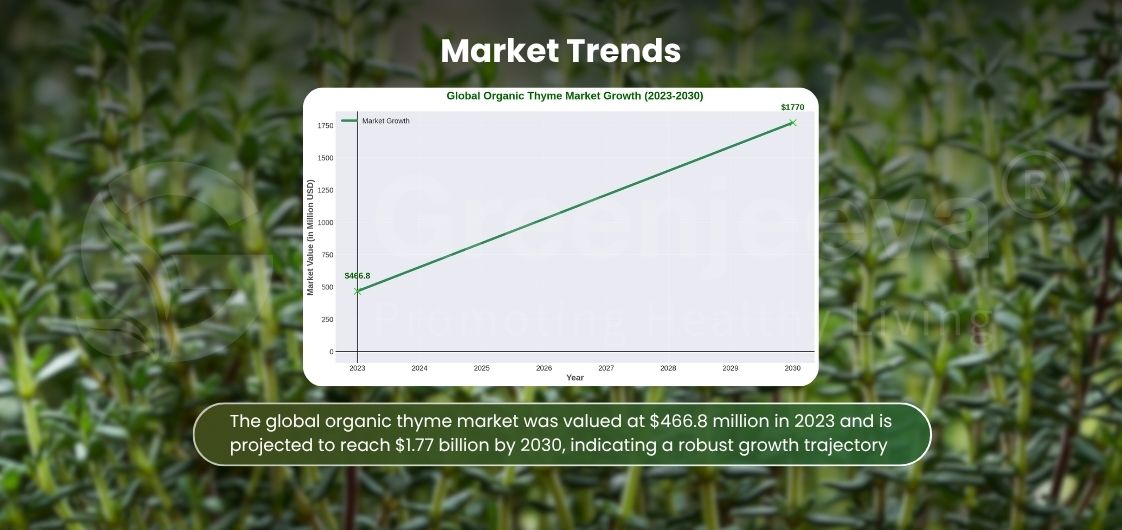
What Are the Emerging Industrial Uses of Organic Thyme Leaf Powder in Canada?
Emerging applications include functional beverages, herbal blends, natural cosmetics, and pet nutrition, reflecting growing demand for clean-label, versatile thyme powder.
– Functional Foods and Beverages – Increasing use for flavor and clean-label positioning in powdered blends, herbal teas, and ready-to-drink mixes.
– Cosmetic Formulations – Integration into natural skincare products for texture, fragrance, and color.
– Pet Nutrition – Added to plant-based or dry formulations for consistency and labeling clarity.
– R&D and Formulation Innovation – Some manufacturers use the powder as a base for multi-herbal blends to meet clean-label demands.
Understanding these emerging uses allows procurement teams to anticipate demand and align bulk purchasing with future R&D and product development projects.
Conclusion
Sourcing Organic Thyme Leaf Powder in Canada requires attention to certifications, documentation, moisture content, bulk supply options, clean-label compatibility, and supplier reliability. By focusing on these practical considerations, procurement managers and R&D teams can secure a high-quality, compliant ingredient supply that supports industrial-scale formulations across multiple markets.
For Canadian manufacturers seeking consistent bulk Organic Thyme Leaf Powder supply, Green Jeeva provides comprehensive documentation, verified certifications, and support for procurement teams to streamline sourcing and formulation workflows. Contact Green Jeeva to discuss bulk supply options and specifications.
**The Food and Drug Administration has not evaluated these statements. This product is not intended to diagnose, treat, cure, or prevent any disease.**